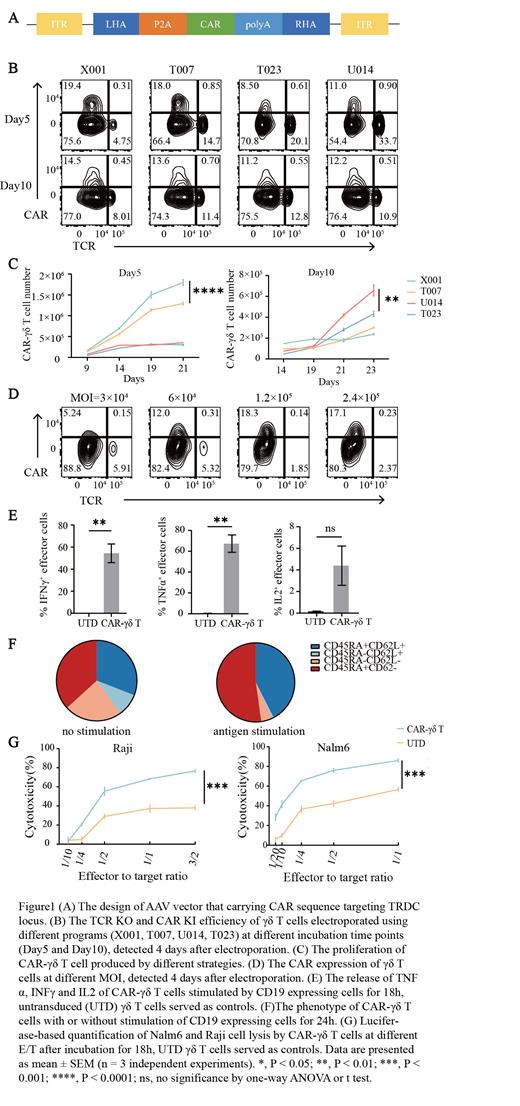
Chimeric antigen receptor (CAR)-γδ T cells are promising in immunotherapy owing to the dual recognition system of γδ T cells, which is potential to exhibit stronger anti-tumor effect. Up to date, established CAR-γδ T cells exhibit poor anti-tumor efficiency and are commonly generated using lentiviral (LV) or gamma-retroviral (γRV) vectors, which may increase the risk of insertional mutagenesis. Nowadays, clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) has been widely used to cleave the genomic sequence precisely, as well as Adeno-associated Virus (AAV) being used to deliver DNA templates for target sequence knock-in (KI) mediated by homology-directed repair, which could contribute to achieve the accurate insertion of CAR at a specific site. Furthermore, the endogenous promoter of TCR has been used to express CAR molecules with stable expression and better antitumor function in the production of CAR-αβ T cells. In the present study, we established and optimized the strategy to generate CAR-γδ T cells, combining the site-specific knock-out (KO) targeting the TCR delta chain constant region (TRDC) site of γδ T cells using CRISPR/Cas9 and site-specific CAR knock-in using AAV.
Firstly, we screened out a functional guide RNA (gRNA) targeting the γδ TCR TRDC locus by prediction tools and validation in cell line model experiments, and achieved an INDEL frequency of 31.8%, which showed a high activity of gRNA. Then, we optimized the option to increase KO efficiency including exploring appropriate Cas9 amount, ratio of gRNA to Cas9 and cell number titration. Besides, we designed AAV vector carrying CAR sequence targeting the gRNA aiming site selected above (Figure1A). After that, we used CRISPR/Cas9 and AAV to knock out TCR and knock in CAR sequence in γδ T cells. We tried different electroporation procedures (X001, T007, U014, T023) and cell culture conditions (cultured in medium containing zoledronic acid (ZOL)/ IL2 for 5 days or for 10 days) to select out the optimal procedure to acquire high KO/KI efficiency and high cell proliferation rate. When we used PBMC being cultured in medium containing zoledronic acid (ZOL)/ IL2 for 5 days (Day 5) to generate CAR-γδ T cells, cells electroporated using X001 procedure showed the best TCR KO efficiency of ~80%(p<0.05)and CAR expression of ~20% (p< 0.01), as well as keeping the highest cell proliferation rate than other procedures (p<0.0001) (Figure 1B and 1C). If we used PBMC being cultured in medium containing zoledronic acid (ZOL)/ IL2 for 10 days (Day 10) to generate CAR-γδ T cells, U014 program also exhibited a relative high CAR expression and the significant quicker cell growth than others in the later period (p<0.01) (Figure 1B and 1C). However, the later proliferation rate of CAR-γδ T cells generated at Day 5 was superior to that generated at Day10. Thus, we determined that a 5 days' cell culture and the use of X001 program were the optimal condition to generate CAR-γδ T cells. Besides, we found that KI efficiency is positively correlative with Multiplicity of infection (MOI). While MOI comes to 1.2×10 5, the KI efficiency couldn't be increased (Figure 1D). Finally, we evaluated the anti-tumor function of CAR-γδ T cells . Antigen stimulated CAR-γδ T cells significantly released TNFα and INFγ, but failed to produce IL2 (Figure 1E). Besides, CAR-γδ T cells could significantly lysis different tumor cells even at a low effector/target ratio (E/T) (Figure 1G). Interestingly, the proportion of memory phenotype (CD62L + ) in CAR-γδ T cells after being stimulated was comparable to that of CAR-γδ T cells without stimulation, indicating the potential of the great anti-tumor activity of CAR-γδ T cells after being stimulated in tumor environment (Figure 1F).
Our new method successfully generated CAR-γδ T cells with a CAR expression of 20% and in a high proliferation rate, which was determined to exert effective anti-tumor effects.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal